Which Statements About The Makeup Of Biological Membranes Are True?

In biology and biochemistry, a lipid is a macro biomolecule that is soluble in nonpolar solvents.[iii] Non-polar solvents are typically hydrocarbons used to deliquesce other naturally occurring hydrocarbon lipid molecules that exercise not (or do non easily) dissolve in water, including fatty acids, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E, and K), monoglycerides, diglycerides, triglycerides, and phospholipids.
The functions of lipids include storing energy, signaling, and acting as structural components of prison cell membranes.[4] [five] Lipids have applications in the corrective and food industries as well as in nanotechnology.[6]
Scientists sometimes define lipids as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to grade structures such as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "edifice-blocks": ketoacyl and isoprene groups.[4] Using this approach, lipids may be divided into eight categories: fatty acids, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensation of ketoacyl subunits); and sterol lipids and prenol lipids (derived from condensation of isoprene subunits).[4]
Although the term "lipid" is sometimes used every bit a synonym for fats, fats are a subgroup of lipids chosen triglycerides. Lipids also cover molecules such as fatty acids and their derivatives (including tri-, di-, monoglycerides, and phospholipids), every bit well every bit other sterol-containing metabolites such as cholesterol.[7] Although humans and other mammals utilize various biosynthetic pathways both to break downwardly and to synthesize lipids, some essential lipids can't be made this way and must exist obtained from the diet.
History [edit]
Lipids may be regarded as organic substances relatively insoluble in h2o, soluble in organic solvents (booze, ether etc.) actually or potentially related to a fat acid and utilized by living cells.
In 1815, Henri Braconnot classified lipids (graisses) in two categories, suifs (solid greases or tallow) and huiles (fluid oils).[viii] In 1823, Michel Eugène Chevreul developed a more detailed classification, including oils, greases, tallow, waxes, resins, balsams and volatile oils (or essential oils).[nine] [10] [11]
The first synthetic triglyceride was reported by Théophile-Jules Pelouze in 1844, when he produced tributyrin by treating butyric acid with glycerin in the presence of concentrated sulfuric acid.[12] Several years after, Marcellin Berthelot, i of Pelouze's students, synthesized tristearin and tripalmitin by reaction of the analogous fatty acids with glycerin in the presence of gaseous hydrogen chloride at high temperature.[thirteen]
In 1827, William Prout recognized fat ("oily" alimentary matters), along with protein ("albuminous") and sugar ("saccharine"), equally an important nutrient for humans and animals.[14] [xv]
For a century, chemists regarded "fats" as only simple lipids fabricated of fatty acids and glycerol (glycerides), only new forms were described later. Theodore Gobley (1847) discovered phospholipids in mammalian brain and hen egg, called by him as "lecithins". Thudichum discovered in human brain some phospholipids (cephalin), glycolipids (cerebroside) and sphingolipids (sphingomyelin).[10]
The terms lipoid, lipin, lipide and lipid have been used with varied meanings from author to author.[16] In 1912, Rosenbloom and Gies proposed the substitution of "lipoid" by "lipin".[17] In 1920, Bloor introduced a new classification for "lipoids": elementary lipoids (greases and waxes), chemical compound lipoids (phospholipoids and glycolipoids), and the derived lipoids (fatty acids, alcohols, sterols).[xviii] [nineteen]
The word lipide, which stems etymologically from Greek λίπος, lipos 'fatty', was introduced in 1923 by the French pharmacologist Gabriel Bertrand.[20] Bertrand included in the concept not only the traditional fats (glycerides), but also the "lipoids", with a circuitous constitution.[10] The word lipide was unanimously canonical by the international commission of the Société de Chimie Biologique during the plenary session on July iii, 1923. The discussion lipide was later anglicized every bit lipid considering of its pronunciation ('lɪpɪd). In French, the suffix -ide, from Ancient Greek -ίδης (meaning 'son of' or 'descendant of'), is always pronounced (ɪd).
In 1947, T. P. Hilditch divers "simple lipids" as greases and waxes (true waxes, sterols, alcohols).
Categories [edit]
Lipids have been classified into eight categories by the Lipid MAPS consortium[iv] as follows:
Fatty acids [edit]


Fatty acids, or fat acid residues when they are function of a lipid, are a various group of molecules synthesized by chain-elongation of an acetyl-CoA primer with malonyl-CoA or methylmalonyl-CoA groups in a process chosen fatty acrid synthesis.[21] [22] They are made of a hydrocarbon chain that terminates with a carboxylic acid grouping; this arrangement confers the molecule with a polar, hydrophilic end, and a nonpolar, hydrophobic stop that is insoluble in h2o. The fatty acid structure is one of the most fundamental categories of biological lipids and is commonly used as a building-block of more structurally complex lipids. The carbon chain, typically betwixt four and 24 carbons long,[23] may exist saturated or unsaturated, and may be attached to functional groups containing oxygen, halogens, nitrogen, and sulfur. If a fatty acid contains a double bail, there is the possibility of either a cis or trans geometric isomerism, which significantly affects the molecule'southward configuration. Cis-double bonds cause the fatty acrid chain to bend, an effect that is compounded with more double bonds in the chain. Iii double bonds in 18-carbon linolenic acid, the almost abundant fat-acyl chains of constitute thylakoid membranes, render these membranes highly fluid despite environmental low-temperatures,[24] and besides makes linolenic acrid give dominating sharp peaks in high resolution thirteen-C NMR spectra of chloroplasts. This in turn plays an of import role in the structure and office of cell membranes.[25] : 193–five Almost naturally occurring fatty acids are of the cis configuration, although the trans form does be in some natural and partially hydrogenated fats and oils.[26]
Examples of biologically of import fatty acids include the eicosanoids, derived primarily from arachidonic acid and eicosapentaenoic acid, that include prostaglandins, leukotrienes, and thromboxanes. Docosahexaenoic acrid is also important in biological systems, peculiarly with respect to sight.[27] [28] Other major lipid classes in the fatty acid category are the fat esters and fatty amides. Fat esters include important biochemical intermediates such as wax esters, fatty acid thioester coenzyme A derivatives, fatty acrid thioester ACP derivatives and fatty acrid carnitines. The fatty amides include N-acyl ethanolamines, such equally the cannabinoid neurotransmitter anandamide.[29]
Glycerolipids [edit]

Glycerolipids are composed of mono-, di-, and tri-substituted glycerols,[30] the best-known being the fatty acid triesters of glycerol, called triglycerides. The discussion "triacylglycerol" is sometimes used synonymously with "triglyceride". In these compounds, the three hydroxyl groups of glycerol are each esterified, typically past different fatty acids. Considering they function as an energy store, these lipids comprise the bulk of storage fatty in beast tissues. The hydrolysis of the ester bonds of triglycerides and the release of glycerol and fatty acids from adipose tissue are the initial steps in metabolizing fatty.[31] : 630–1
Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more than sugar residues attached to glycerol via a glycosidic linkage. Examples of structures in this category are the digalactosyldiacylglycerols institute in plant membranes[32] and seminolipid from mammalian sperm cells.[33]
Glycerophospholipids [edit]
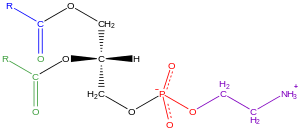
Glycerophospholipids, usually referred to as phospholipids (though sphingomyelins are also classified every bit phospholipids), are ubiquitous in nature and are key components of the lipid bilayer of cells,[34] too as existence involved in metabolism and cell signaling.[35] Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their limerick has been implicated in diverse neurological disorders.[36] Glycerophospholipids may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-iii position of the glycerol backbone in eukaryotes and eubacteria, or the sn-ane position in the case of archaebacteria.[37]
Examples of glycerophospholipids constitute in biological membranes are phosphatidylcholine (also known every bit PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving equally a main component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of or, themselves, membrane-derived 2d messengers.[31] : 844 Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, only there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.[38]
Sphingolipids [edit]
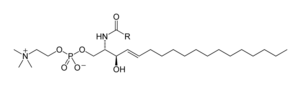
Sphingolipids are a complicated family of compounds[39] that share a common structural feature, a sphingoid base backbone that is synthesized de novo from the amino acid serine and a long-chain fatty acyl CoA, and so converted into ceramides, phosphosphingolipids, glycosphingolipids and other compounds. The major sphingoid base of mammals is ordinarily referred to equally sphingosine. Ceramides (Northward-acyl-sphingoid bases) are a major subclass of sphingoid base derivatives with an amide-linked fat acrid. The fatty acids are typically saturated or mono-unsaturated with chain lengths from 16 to 26 carbon atoms.[25] : 421–two
The major phosphosphingolipids of mammals are sphingomyelins (ceramide phosphocholines),[forty] whereas insects contain mainly ceramide phosphoethanolamines[41] and fungi accept phytoceramide phosphoinositols and mannose-containing headgroups.[42] The glycosphingolipids are a diverse family of molecules composed of one or more carbohydrate residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides.
Sterols [edit]

Sterols, such equally cholesterol and its derivatives, are an of import component of membrane lipids,[43] along with the glycerophospholipids and sphingomyelins. Other examples of sterols are the bile acids and their conjugates,[44] which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver. The found equivalents are the phytosterols, such as β-sitosterol, stigmasterol, and brassicasterol; the latter compound is too used as a biomarker for algal growth.[45] The predominant sterol in fungal jail cell membranes is ergosterol.[46]
Sterols are steroids in which ane of the hydrogen atoms is substituted with a hydroxyl group, at position 3 in the carbon chain. They have in mutual with steroids the aforementioned fused iv-ring core construction. Steroids have dissimilar biological roles as hormones and signaling molecules. The xviii-carbon (C18) steroids include the estrogen family whereas the C19 steroids comprise the androgens such equally testosterone and androsterone. The C21 subclass includes the progestogens also as the glucocorticoids and mineralocorticoids.[2] : 749 The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring of the core structure.[47]
Prenols [edit]

Prenol lipid (2E-geraniol)
Prenol lipids are synthesized from the five-carbon-unit precursors isopentenyl diphosphate and dimethylallyl diphosphate, which are produced mainly via the mevalonic acid (MVA) pathway.[48] The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed past the successive addition of C5 units, and are classified according to number of these terpene units. Structures containing greater than twoscore carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that role as antioxidants and as precursors of vitamin A.[49] Some other biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid cadre of non-isoprenoid origin.[50] Vitamin Due east and vitamin Thou, equally well equally the ubiquinones, are examples of this class. Prokaryotes synthesize polyprenols (called bactoprenols) in which the last isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced.[51]
Saccharolipids [edit]
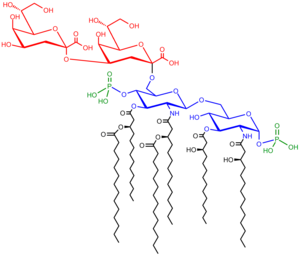
Construction of the saccharolipid Kdotwo-lipid A.[52] Glucosamine residues in blue, Kdo residues in cherry, acyl chains in black and phosphate groups in green.
Saccharolipids depict compounds in which fatty acids are linked to a sugar backbone, forming structures that are uniform with membrane bilayers. In the saccharolipids, a monosaccharide substitutes for the glycerol backbone present in glycerolipids and glycerophospholipids. The well-nigh familiar saccharolipids are the acylated glucosamine precursors of the Lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many every bit seven fatty-acyl bondage. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.[52]
Polyketides [edit]
Polyketides are synthesized by polymerization of acetyl and propionyl subunits by classic enzymes as well as iterative and multimodular enzymes that share mechanistic features with the fatty acid synthases. They comprise many secondary metabolites and natural products from beast, institute, bacterial, fungal and marine sources, and have great structural diversity.[53] [54] Many polyketides are cyclic molecules whose backbones are oft further modified by glycosylation, methylation, hydroxylation, oxidation, or other processes. Many commonly used anti-microbial, anti-parasitic, and anti-cancer agents are polyketides or polyketide derivatives, such as erythromycins, tetracyclines, avermectins, and antitumor epothilones.[55]
Biological functions [edit]
Component of biological membranes [edit]
Eukaryotic cells feature the compartmentalized membrane-bound organelles that carry out unlike biological functions. The glycerophospholipids are the main structural component of biological membranes, every bit the cellular plasma membrane and the intracellular membranes of organelles; in animal cells, the plasma membrane physically separates the intracellular components from the extracellular environment.[ citation needed ] The glycerophospholipids are amphipathic molecules (containing both hydrophobic and hydrophilic regions) that incorporate a glycerol core linked to two fatty acid-derived "tails" past ester linkages and to ane "head" group by a phosphate ester linkage.[ commendation needed ] While glycerophospholipids are the major component of biological membranes, other non-glyceride lipid components such equally sphingomyelin and sterols (mainly cholesterol in animal cell membranes) are likewise found in biological membranes.[56] [2] : 329–331 In plants and algae, the galactosyldiacylglycerols,[57] and sulfoquinovosyldiacylglycerol,[32] which lack a phosphate group, are important components of membranes of chloroplasts and related organelles and are the about arable lipids in photosynthetic tissues, including those of higher plants, algae and certain bacteria.[ commendation needed ]
Plant thylakoid membranes have the largest lipid component of a non-bilayer forming monogalactosyl diglyceride (MGDG), and little phospholipids; despite this unique lipid composition, chloroplast thylakoid membranes have been shown to contain a dynamic lipid-bilayer matrix as revealed by magnetic resonance and electron microscope studies.[58]
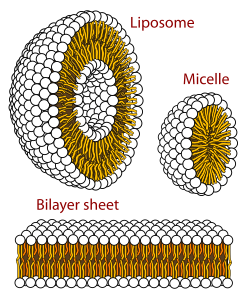
A biological membrane is a form of lamellar stage lipid bilayer. The formation of lipid bilayers is an energetically preferred process when the glycerophospholipids described above are in an aqueous environment.[2] : 333–4 This is known equally the hydrophobic effect. In an aqueous system, the polar heads of lipids align towards the polar, aqueous surround, while the hydrophobic tails minimize their contact with h2o and tend to cluster together, forming a vesicle; depending on the concentration of the lipid, this biophysical interaction may result in the germination of micelles, liposomes, or lipid bilayers. Other aggregations are too observed and class office of the polymorphism of amphiphile (lipid) behavior. Phase behavior is an area of study within biophysics and is the subject of current[ when? ] academic research.[59] [threescore] Micelles and bilayers class in the polar medium by a process known every bit the hydrophobic outcome.[61] When dissolving a lipophilic or amphiphilic substance in a polar environment, the polar molecules (i.east., water in an aqueous solution) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile. So in an aqueous surroundings, the water molecules form an ordered "clathrate" muzzle effectually the dissolved lipophilic molecule.[62]
The germination of lipids into protocell membranes represents a key step in models of abiogenesis, the origin of life.[63]
Free energy storage [edit]
Triglycerides, stored in adipose tissue, are a major class of energy storage both in animals and plants. They are a major 'source' of energy in aerobic respiration because they release the energy of twice more dioxygen than carbohydrates such every bit glycogen do, per mass; this is due to the relatively low oxygen content of triglycerides.[64] The complete oxidation of fatty acids releases about 38 kJ/g (ix kcal/chiliad), compared with only 17 kJ/g (4 kcal/one thousand) for the oxidative breakdown of carbohydrates and proteins. The adipocyte, or fat cell, is designed for continuous synthesis and breakdown of triglycerides in animals, with breakdown controlled mainly by the activation of hormone-sensitive enzyme lipase.[65] Migratory birds that must fly long distances without eating apply triglycerides to fuel their flights.[ii] : 619
Signaling [edit]
Show has emerged showing that lipid signaling is a vital office of the cell signaling.[66] [67] [68] [69] Lipid signaling may occur via activation of G protein-coupled or nuclear receptors, and members of several different lipid categories have been identified as signaling molecules and cellular messengers.[lxx] These include sphingosine-ane-phosphate, a sphingolipid derived from ceramide that is a potent messenger molecule involved in regulating calcium mobilization,[71] cell growth, and apoptosis;[72] diacylglycerol (DAG) and the phosphatidylinositol phosphates (PIPs), involved in calcium-mediated activation of protein kinase C;[73] the prostaglandins, which are one type of fatty-acid derived eicosanoid involved in inflammation and immunity;[74] the steroid hormones such every bit estrogen, testosterone and cortisol, which modulate a host of functions such equally reproduction, metabolism and claret force per unit area; and the oxysterols such as 25-hydroxy-cholesterol that are liver X receptor agonists.[75] Phosphatidylserine lipids are known to exist involved in signaling for the phagocytosis of apoptotic cells or pieces of cells. They accomplish this past being exposed to the extracellular face up of the cell membrane after the inactivation of flippases which place them exclusively on the cytosolic side and the activation of scramblases, which scramble the orientation of the phospholipids. Subsequently this occurs, other cells recognize the phosphatidylserines and phagocytosize the cells or cell fragments exposing them.[76]
Other functions [edit]
The "fat-soluble" vitamins (A, D, E and K) – which are isoprene-based lipids – are essential nutrients stored in the liver and fat tissues, with a diverse range of functions. Acyl-carnitines are involved in the ship and metabolism of fatty acids in and out of mitochondria, where they undergo beta oxidation.[77] Polyprenols and their phosphorylated derivatives besides play important transport roles, in this instance the transport of oligosaccharides beyond membranes. Polyprenol phosphate sugars and polyprenol diphosphate sugars function in actress-cytoplasmic glycosylation reactions, in extracellular polysaccharide biosynthesis (for example, peptidoglycan polymerization in bacteria), and in eukaryotic poly peptide N-glycosylation.[78] [79] Cardiolipins are a bracket of glycerophospholipids containing iv acyl chains and three glycerol groups that are especially abundant in the inner mitochondrial membrane.[80] [81] They are believed to actuate enzymes involved with oxidative phosphorylation.[82] Lipids too form the ground of steroid hormones.[83]
Metabolism [edit]
The major dietary lipids for humans and other animals are animal and plant triglycerides, sterols, and membrane phospholipids. The procedure of lipid metabolism synthesizes and degrades the lipid stores and produces the structural and functional lipids characteristic of individual tissues.
Biosynthesis [edit]
In animals, when there is an oversupply of dietary carbohydrate, the excess carbohydrate is converted to triglycerides. This involves the synthesis of fatty acids from acetyl-CoA and the esterification of fatty acids in the product of triglycerides, a process called lipogenesis.[two] : 634 Fatty acids are made by fatty acrid synthases that polymerize and and then reduce acetyl-CoA units. The acyl bondage in the fatty acids are extended by a wheel of reactions that add the acetyl group, reduce information technology to an alcohol, dehydrate it to an alkene group and and so reduce information technology once more to an alkane group. The enzymes of fatty acrid biosynthesis are divided into ii groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional protein,[84] while in plant plastids and bacteria separate enzymes perform each step in the pathway.[85] [86] The fat acids may be subsequently converted to triglycerides that are packaged in lipoproteins and secreted from the liver.
The synthesis of unsaturated fatty acids involves a desaturation reaction, whereby a double bond is introduced into the fatty acyl chain. For example, in humans, the desaturation of stearic acid by stearoyl-CoA desaturase-1 produces oleic acid. The doubly unsaturated fat acid linoleic acid as well as the triply unsaturated α-linolenic acid cannot be synthesized in mammalian tissues, and are therefore essential fatty acids and must be obtained from the diet.[2] : 643
Triglyceride synthesis takes place in the endoplasmic reticulum by metabolic pathways in which acyl groups in fatty acyl-CoAs are transferred to the hydroxyl groups of glycerol-3-phosphate and diacylglycerol.[2] : 733–nine
Terpenes and isoprenoids, including the carotenoids, are made past the associates and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate.[48] These precursors tin be fabricated in dissimilar ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA,[87] while in plants and leaner the non-mevalonate pathway uses pyruvate and glyceraldehyde three-phosphate as substrates.[48] [88] One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene and then folded up and formed into a fix of rings to make lanosterol.[89] Lanosterol can then exist converted into other steroids such equally cholesterol and ergosterol.[89] [ninety]
Deposition [edit]
Beta oxidation is the metabolic process by which fatty acids are cleaved down in the mitochondria or in peroxisomes to generate acetyl-CoA. For the most part, fat acids are oxidized by a mechanism that is similar to, just non identical with, a reversal of the process of fatty acrid synthesis. That is, two-carbon fragments are removed sequentially from the carboxyl end of the acid later steps of dehydrogenation, hydration, and oxidation to form a beta-keto acid, which is split by thiolysis. The acetyl-CoA is so ultimately converted into ATP, COtwo, and HtwoO using the citric acrid wheel and the electron transport chain. Hence the citric acid cycle can start at acetyl-CoA when fat is being broken downwardly for energy if there is little or no glucose bachelor. The energy yield of the consummate oxidation of the fatty acid palmitate is 106 ATP.[two] : 625–6 Unsaturated and odd-chain fatty acids require additional enzymatic steps for degradation.
Nutrition and wellness [edit]
About of the fatty constitute in food is in the class of triglycerides, cholesterol, and phospholipids. Some dietary fat is necessary to facilitate absorption of fat-soluble vitamins (A, D, Eastward, and K) and carotenoids.[91] : 903 Humans and other mammals have a dietary requirement for certain essential fatty acids, such as linoleic acid (an omega-6 fat acid) and blastoff-linolenic acid (an omega-three fat acid) because they cannot be synthesized from simple precursors in the nutrition.[2] : 643 Both of these fatty acids are 18-carbon polyunsaturated fatty acids differing in the number and position of the double bonds. Near vegetable oils are rich in linoleic acid (safflower, sunflower, and corn oils). Blastoff-linolenic acid is found in the green leaves of plants and in some seeds, nuts, and legumes (in particular flax, rapeseed, walnut, and soy).[92] Fish oils are particularly rich in the longer-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA).[91] : 388 Many studies have shown positive health benefits associated with consumption of omega-3 fat acids on babe development, cancer, cardiovascular diseases, and various mental illnesses (such as depression, attention-arrears hyperactivity disorder, and dementia).[93] [94]
In contrast, it is at present well-established that consumption of trans fats, such as those nowadays in partially hydrogenated vegetable oils, are a gamble cistron for cardiovascular disease. Fats that are skilful for 1 may exist turned into trans fats by improper cooking methods that outcome in overcooking the lipids.[95] [96] [97]
A few studies have suggested that full dietary fat intake is linked to an increased take a chance of obesity [98] [99] and diabetes;[100] however, a number of very large studies, including the Women's Health Initiative Dietary Modification Trial, an eight-year report of 49,000 women, the Nurses' Health Study, and the Health Professionals Follow-up Study, revealed no such links.[101] [102] None of these studies suggested any connection betwixt percent of calories from fatty and gamble of cancer, heart disease, or weight gain. The Nutrition Source,[103] a website maintained by the department of diet at the T. H. Chan School of Public Wellness at Harvard University, summarizes the current evidence on the upshot of dietary fat: "Detailed research—much of it done at Harvard—shows that the total amount of fat in the diet isn't really linked with weight or disease."[104]
See as well [edit]
- Solid lipid nanoparticle – Novel drug delivery organisation
- Simple lipid
- Emulsion test
- Lipid microdomain
- Membrane lipid – Lipid molecules on cell membrane
- Fat – Esters of 3 fatty acid chains and the alcohol glycerol, one of the iii main macronutrients, also known equally triglycerides
- Lipid signaling
- Lipidomics
- Protein–lipid interaction
- Phenolic lipid, a class of natural products equanimous of long aliphatic bondage and phenolic rings that occur in plants, fungi and bacteria
References [edit]
- ^ Maitland J Jr (1998). Organic Chemistry. Westward W Norton & Co Inc (Np). p. 139. ISBN978-0-393-97378-5.
- ^ a b c d e f m h i j Stryer L, Berg JM, Tymoczko JL (2007). Biochemistry (6th ed.). San Francisco: W.H. Freeman. ISBN978-0-7167-8724-2.
- ^ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gilt Book") (1997). Online corrected version: (2006–) "lipids". doi:10.1351/goldbook.L03571
- ^ a b c d Fahy E, Subramaniam Due south, Murphy RC, Nishijima Chiliad, Raetz CR, Shimizu T, Spener F, van Meer G, Wakelam MJ, Dennis EA (Apr 2009). "Update of the LIPID MAPS comprehensive classification organisation for lipids". Journal of Lipid Research. 50 (S1): S9-14. doi:x.1194/jlr.R800095-JLR200. PMC2674711. PMID 19098281.
- ^ Subramaniam Southward, Fahy Eastward, Gupta S, Sud M, Byrnes RW, Cotter D, Dinasarapu AR, Maurya MR (October 2011). "Bioinformatics and systems biology of the lipidome". Chemical Reviews. 111 (10): 6452–xc. doi:10.1021/cr200295k. PMC3383319. PMID 21939287.
- ^ Mashaghi S, Jadidi T, Koenderink Grand, Mashaghi A (February 2013). "Lipid nanotechnology". International Journal of Molecular Sciences. 14 (two): 4242–82. doi:ten.3390/ijms14024242. PMC3588097. PMID 23429269.

- ^ Michelle A, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD (1993). Human Biology and Health. Englewood Cliffs, New Jersey, United states: Prentice Hall. ISBN978-0-13-981176-0.
- ^ Braconnot H (31 March 1815). "Sur la nature des corps gras". Annales de chimie. ii (XCIII): 225–277.
- ^ Chevreul ME (1823). Recherches sur les corps gras d'origine animale. Paris: Levrault.
- ^ a b c Leray C (2012). Introduction to Lipidomics. Boca Raton: CRC Press. ISBN9781466551466.
- ^ Leray C (2015). "Introduction, History and Development.". Lipids. Nutrition and health. Boca Raton: CRC Press. ISBN9781482242317.
- ^ Pelouze TJ, Gélis A (1844). "Mémoire sur l'acide butyrique". Annales de Chimie et de Physique. 10: 434.
- ^ Comptes rendus hebdomadaires des séances de l'Académie des Sciences, Paris, 1853, 36, 27; Annales de Chimie et de Physique 1854, 41, 216
- ^ Leray C. "Chronological history of lipid center". Cyberlipid Center. Archived from the original on 2017-x-xiii. Retrieved 2017-12-01 .
- ^ Prout W (1827). "On the ultimate composition of simple alimentary substances, with some preliminary remarks on the analysis of organised bodies in general". Phil. Trans.: 355–388.
- ^ Culling CF (1974). "Lipids. (Fats, Lipoids. Lipins).". Handbook of Histopathological Techniques (3rd ed.). London: Butterworths. pp. 351–376. ISBN9781483164793.
- ^ Rosenbloom J, Gies WJ (1911). "Suggestion to teachers of biochemistry. I. A proposed chemical nomenclature of lipins, with a annotation on the intimate relation between cholesterols and bile salts". Biochem. Bull. 1: 51–6.
- ^ Bloor WR (1920). "Outline of a classication of the lipids". Proc. Soc. Exp. Biol. Med. 17 (half-dozen): 138–140. doi:ten.3181/00379727-17-75. S2CID 75844378.
- ^ Christie WW, Han X (2010). Lipid Analysis: Isolation, Separation, Identification and Lipidomic Assay. Bridgwater, England: The Oily Press. ISBN9780857097866.
- ^ Bertrand G (1923). "Projet de reforme de la nomenclature de Chimie biologique". Bulletin de la Société de Chimie Biologique. v: 96–109.
- ^ Vance JE, Vance DE (2002). Biochemistry of Lipids, Lipoproteins and Membranes. Amsterdam: Elsevier. ISBN978-0-444-51139-3.
- ^ Brown HA, ed. (2007). Lipodomics and Bioactive Lipids: Mass Spectrometry Based Lipid Analysis. Methods in Enzymology. Vol. 423. Boston: Bookish Press. ISBN978-0-12-373895-0.
- ^ Hunt SM, Groff JL, Gropper SA (1995). Advanced Diet and Human Metabolism. Belmont, California: W Pub. Co. p. 98. ISBN978-0-314-04467-9.
- ^ Yashroy RC (1987). "13C NMR studies of lipid fatty acyl chains of chloroplast membranes". Indian Periodical of Biochemistry and Biophysics. 24 (6): 177–178.
- ^ a b Devlin TM (1997). Textbook of Biochemistry: With Clinical Correlations (4th ed.). Chichester: John Wiley & Sons. ISBN978-0-471-17053-2.
- ^ Hunter JE (November 2006). "Dietary trans fatty acids: review of contempo human studies and nutrient industry responses". Lipids. 41 (11): 967–92. doi:10.1007/s11745-006-5049-y. PMID 17263298. S2CID 1625062.
- ^ Furse S (2011-12-02). "A Long Lipid, a Long Name: Docosahexaenoic Acid". The Lipid Chronicles.
- ^ "DHA for Optimal Encephalon and Visual Operation". DHA/EPA Omega-3 Institute.
- ^ Fezza F, De Simone C, Amadio D, Maccarrone M (2008). "Fatty acid amide hydrolase: a gate-keeper of the endocannabinoid organization". Lipids in Wellness and Disease. Subcellular Biochemistry. Vol. 49. pp. 101–32. doi:10.1007/978-1-4020-8831-5_4. ISBN978-1-4020-8830-8. PMID 18751909.
- ^ Coleman RA, Lee DP (March 2004). "Enzymes of triacylglycerol synthesis and their regulation". Progress in Lipid Research. 43 (2): 134–76. doi:10.1016/S0163-7827(03)00051-1. PMID 14654091.
- ^ a b van Holde KE, Mathews CK (1996). Biochemistry (second ed.). Menlo Park, California: Benjamin/Cummings Pub. Co. ISBN978-0-8053-3931-4.
- ^ a b Hölzl Grand, Dörmann P (September 2007). "Structure and role of glycoglycerolipids in plants and bacteria". Progress in Lipid Research. 46 (5): 225–43. doi:10.1016/j.plipres.2007.05.001. PMID 17599463.
- ^ Honke K, Zhang Y, Cheng Ten, Kotani Northward, Taniguchi N (2004). "Biological roles of sulfoglycolipids and pathophysiology of their deficiency". Glycoconjugate Journal. 21 (1–2): 59–62. doi:10.1023/B:GLYC.0000043749.06556.3d. PMID 15467400. S2CID 2678053.
- ^ "The Structure of a Membrane". The Lipid Chronicles. 2011-11-05. Retrieved 2011-12-31 .
- ^ Berridge MJ, Irvine RF (September 1989). "Inositol phosphates and cell signalling". Nature. 341 (6239): 197–205. Bibcode:1989Natur.341..197B. doi:10.1038/341197a0. PMID 2550825. S2CID 26822092.
- ^ Farooqui AA, Horrocks LA, Farooqui T (June 2000). "Glycerophospholipids in brain: their metabolism, incorporation into membranes, functions, and involvement in neurological disorders". Chemistry and Physics of Lipids. 106 (1): 1–29. doi:10.1016/S0009-3084(00)00128-6. PMID 10878232.
- ^ Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA (2007). "Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry". Lipidomics and Bioactive Lipids: Mass‐Spectrometry–Based Lipid Analysis. Methods in Enzymology. Vol. 432. pp. 21–57. doi:x.1016/S0076-6879(07)32002-8. ISBN978-0-12-373895-0. PMID 17954212.
- ^ Paltauf F (December 1994). "Ether lipids in biomembranes". Chemical science and Physics of Lipids. 74 (2): 101–39. doi:10.1016/0009-3084(94)90054-10. PMID 7859340.
- ^ Merrill AH, Sandoff K (2002). "Chapter 14: Sphingolipids: Metabolism and Jail cell Signaling" (PDF). In Vance JE, Vance EE (eds.). Biochemistry of Lipids, Lipoproteins and Membranes (4th ed.). Amsterdam: Elsevier. pp. 373–407. ISBN978-0-444-51138-6.
- ^ Hori T, Sugita Thousand (1993). "Sphingolipids in lower animals". Progress in Lipid Enquiry. 32 (one): 25–45. doi:ten.1016/0163-7827(93)90003-F. PMID 8415797.
- ^ Wiegandt H (January 1992). "Insect glycolipids". Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism. 1123 (2): 117–26. doi:10.1016/0005-2760(92)90101-Z. PMID 1739742.
- ^ Guan 10, Wenk MR (May 2008). "Biochemistry of inositol lipids". Frontiers in Bioscience. 13 (13): 3239–51. doi:10.2741/2923. PMID 18508430.
- ^ Bach D, Wachtel Eastward (March 2003). "Phospholipid/cholesterol model membranes: formation of cholesterol crystallites". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1610 (2): 187–97. doi:10.1016/S0005-2736(03)00017-8. PMID 12648773.
- ^ Russell DW (2003). "The enzymes, regulation, and genetics of bile acrid synthesis". Annual Review of Biochemistry. 72: 137–74. doi:10.1146/annurev.biochem.72.121801.161712. PMID 12543708.
- ^ Villinski JC, Hayes JM, Brassell SC, Riggert VL, Dunbar R (2008). "Sedimentary sterols equally biogeochemical indicators in the Southern Body of water". Organic Geochemistry. 39 (5): 567–588. doi:10.1016/j.orggeochem.2008.01.009.
- ^ Deacon J (2005). Fungal Biological science. Cambridge, Massachusetts: Blackwell Publishers. p. 342. ISBN978-ane-4051-3066-0.
- ^ Burgoo R, Verstuyf A, Mathieu C, Van Cromphaut S, Masuyama R, Dehaes P, Carmeliet K (December 2006). "Vitamin D resistance". Best Practise & Research. Clinical Endocrinology & Metabolism. twenty (4): 627–45. doi:10.1016/j.beem.2006.09.008. PMID 17161336.
- ^ a b c Kuzuyama T, Seto H (April 2003). "Diversity of the biosynthesis of the isoprene units". Natural Product Reports. xx (2): 171–83. doi:10.1039/b109860h. PMID 12735695.
- ^ Rao AV, Rao LG (March 2007). "Carotenoids and human health". Pharmacological Inquiry. 55 (3): 207–xvi. doi:10.1016/j.phrs.2007.01.012. PMID 17349800.
- ^ Brunmark A, Cadenas Eastward (1989). "Redox and add-on chemistry of quinoid compounds and its biological implications". Free Radical Biology & Medicine. 7 (4): 435–77. doi:10.1016/0891-5849(89)90126-3. PMID 2691341.
- ^ Swiezewska E, Danikiewicz W (July 2005). "Polyisoprenoids: structure, biosynthesis and role". Progress in Lipid Research. 44 (four): 235–58. doi:ten.1016/j.plipres.2005.05.002. PMID 16019076.
- ^ a b Raetz CR, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC, et al. (May 2006). "Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4". Journal of Lipid Inquiry. 47 (5): 1097–111. doi:10.1194/jlr.M600027-JLR200. hdl:10919/74310. PMID 16479018.

- ^ Walsh CT (March 2004). "Polyketide and nonribosomal peptide antibiotics: modularity and versatility". Science. 303 (5665): 1805–10. Bibcode:2004Sci...303.1805W. doi:x.1126/science.1094318. PMID 15031493. S2CID 44858908.
- ^ Caffrey P, Aparicio JF, Malpartida F, Zotchev SB (2008). "Biosynthetic engineering of polyene macrolides towards generation of improved antifungal and antiparasitic agents". Current Topics in Medicinal Chemical science. 8 (viii): 639–53. doi:10.2174/156802608784221479. PMID 18473889.
- ^ Minto RE, Blacklock BJ (July 2008). "Biosynthesis and function of polyacetylenes and allied natural products". Progress in Lipid Enquiry. 47 (4): 233–306. doi:ten.1016/j.plipres.2008.02.002. PMC2515280. PMID 18387369.
- ^ Coones RT, Green RJ, Frazier RA (July 2021). "Investigating lipid headgroup composition within epithelial membranes: a systematic review". Soft Affair. 17 (28): 6773–6786. Bibcode:2021SMat...17.6773C. doi:10.1039/D1SM00703C. ISSN 1744-683X. PMID 34212942. S2CID 235708094.
- ^ Heinz E. (1996). "Plant glycolipids: structure, isolation and assay", pp. 211–332 in Advances in Lipid Methodology, Vol. 3. W.W. Christie (ed.). Oily Press, Dundee. ISBN 978-0-9514171-vi-iv
- ^ Yashroy RC (1990). "Magnetic resonance studies of dynamic organisation of lipids in chloroplast membranes". Journal of Biosciences. 15 (4): 281–288. doi:10.1007/BF02702669. S2CID 360223.
- ^ van Meer G, Voelker DR, Feigenson GW (February 2008). "Membrane lipids: where they are and how they behave". Nature Reviews Molecular Cell Biology. nine (2): 112–24. doi:x.1038/nrm2330. PMC2642958. PMID 18216768.
- ^ Feigenson GW (November 2006). "Phase beliefs of lipid mixtures". Nature Chemical Biological science. 2 (11): 560–iii. doi:ten.1038/nchembio1106-560. PMC2685072. PMID 17051225.
- ^ Wiggins PM (Dec 1990). "Role of h2o in some biological processes". Microbiological Reviews. 54 (4): 432–49. doi:10.1128/MMBR.54.iv.432-449.1990. PMC372788. PMID 2087221.
- ^ Raschke TM, Levitt M (May 2005). "Nonpolar solutes enhance h2o construction within hydration shells while reducing interactions betwixt them". Proceedings of the National Academy of Sciences of the United States of America. 102 (xix): 6777–82. doi:10.1073/pnas.0500225102. PMC1100774. PMID 15867152.
- ^ Segré D, Ben-Eli D, Deamer DW, Lancet D (2001). "The lipid world" (PDF). Origins of Life and Evolution of the Biosphere. 31 (1–2): 119–45. Bibcode:2001OLEB...31..119S. doi:10.1023/A:1006746807104. PMID 11296516. S2CID 10959497.
- ^ Rosen ED, Spiegelman BM (December 2006). "Adipocytes as regulators of free energy residuum and glucose homeostasis". Nature. 444 (7121): 847–53. Bibcode:2006Natur.444..847R. doi:ten.1038/nature05483. PMC3212857. PMID 17167472.
- ^ Brasaemle DL (December 2007). "Thematic review serial: adipocyte biology. The perilipin family unit of structural lipid droplet proteins: stabilization of lipid aerosol and control of lipolysis". Periodical of Lipid Inquiry. 48 (12): 2547–59. doi:ten.1194/jlr.R700014-JLR200. PMID 17878492.
- ^ Malinauskas T, Aricescu AR, Lu W, Siebold C, Jones EY (July 2011). "Modular machinery of Wnt signaling inhibition by Wnt inhibitory factor 1". Nature Structural & Molecular Biology. 18 (8): 886–93. doi:ten.1038/nsmb.2081. PMC3430870. PMID 21743455.
- ^ Malinauskas T (March 2008). "Docking of fatty acids into the WIF domain of the human Wnt inhibitory gene-ane". Lipids. 43 (iii): 227–30. doi:ten.1007/s11745-007-3144-iii. PMID 18256869. S2CID 31357937.
- ^ Wang Ten (June 2004). "Lipid signaling". Current Opinion in Plant Biology. seven (iii): 329–36. doi:x.1016/j.pbi.2004.03.012. PMID 15134755.
- ^ Dinasarapu AR, Saunders B, Ozerlat I, Azam M, Subramaniam S (June 2011). "Signaling gateway molecule pages--a information model perspective". Bioinformatics. 27 (12): 1736–viii. doi:x.1093/bioinformatics/btr190. PMC3106186. PMID 21505029.
- ^ Eyster KM (March 2007). "The membrane and lipids as integral participants in point transduction: lipid signal transduction for the non-lipid biochemist". Advances in Physiology Didactics. 31 (1): 5–16. doi:10.1152/advan.00088.2006. PMID 17327576. S2CID 9194419.
- ^ Hinkovska-Galcheva V, VanWay SM, Shanley TP, Kunkel RG (November 2008). "The function of sphingosine-1-phosphate and ceramide-1-phosphate in calcium homeostasis". Current Opinion in Investigational Drugs. 9 (xi): 1192–205. PMID 18951299.
- ^ Saddoughi SA, Song P, Ogretmen B (2008). "Roles of bioactive sphingolipids in cancer biological science and therapeutics". Lipids in Health and Illness. Subcellular Biochemistry. Vol. 49. pp. 413–40. doi:x.1007/978-1-4020-8831-5_16. ISBN978-ane-4020-8830-eight. PMC2636716. PMID 18751921.
- ^ Klein C, Malviya AN (Jan 2008). "Mechanism of nuclear calcium signaling by inositol 1,four,v-trisphosphate produced in the nucleus, nuclear located protein kinase C and cyclic AMP-dependent poly peptide kinase". Frontiers in Bioscience. 13 (thirteen): 1206–26. doi:10.2741/2756. PMID 17981624.
- ^ Boyce JA (August 2008). "Eicosanoids in asthma, allergic inflammation, and host defence". Current Molecular Medicine. 8 (5): 335–49. doi:10.2174/156652408785160989. PMID 18691060.
- ^ Bełtowski J (2008). "Liver X receptors (LXR) as therapeutic targets in dyslipidemia". Cardiovascular Therapeutics. 26 (4): 297–316. doi:10.1111/j.1755-5922.2008.00062.x. PMID 19035881.
- ^ Biermann Grand, Maueröder C, Brauner JM, Chaurio R, Janko C, Herrmann 1000, Muñoz LE (December 2013). "Surface code--biophysical signals for apoptotic cell clearance". Physical Biology. ten (half dozen): 065007. Bibcode:2013PhBio..10f5007B. doi:10.1088/1478-3975/x/half dozen/065007. PMID 24305041.
- ^ Indiveri C, Tonazzi A, Palmieri F (October 1991). "Characterization of the unidirectional transport of carnitine catalyzed past the reconstituted carnitine carrier from rat liver mitochondria". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1069 (1): 110–6. doi:10.1016/0005-2736(91)90110-t. PMID 1932043.
- ^ Parodi AJ, Leloir LF (April 1979). "The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell". Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 559 (1): 1–37. doi:x.1016/0304-4157(79)90006-6. PMID 375981.
- ^ Helenius A, Aebi Thousand (March 2001). "Intracellular functions of Due north-linked glycans". Scientific discipline. 291 (5512): 2364–nine. Bibcode:2001Sci...291.2364H. doi:10.1126/science.291.5512.2364. PMID 11269317. S2CID 7277949.
- ^ Nowicki Thousand, Müller F, Frentzen M (April 2005). "Cardiolipin synthase of Arabidopsis thaliana". FEBS Letters. 579 (10): 2161–5. doi:ten.1016/j.febslet.2005.03.007. PMID 15811335. S2CID 21937549.
- ^ Gohil VM, Greenberg ML (February 2009). "Mitochondrial membrane biogenesis: phospholipids and proteins go hand in manus". The Journal of Cell Biology. 184 (four): 469–72. doi:10.1083/jcb.200901127. PMC2654137. PMID 19237595.
- ^ Hoch FL (March 1992). "Cardiolipins and biomembrane function" (PDF). Biochimica et Biophysica Acta (BBA) - Reviews on Biomembranes. 1113 (ane): 71–133. doi:10.1016/0304-4157(92)90035-ix. hdl:2027.42/30145. PMID 1550861.
- ^ "Steroids". Elmhurst. edu. Archived from the original on 2011-10-23. Retrieved 2013-10-x .
- ^ Chirala SS, Wakil SJ (November 2004). "Structure and function of fauna fat acid synthase". Lipids. 39 (11): 1045–53. doi:10.1007/s11745-004-1329-9. PMID 15726818. S2CID 4043407.
- ^ White SW, Zheng J, Zhang YM (2005). "The structural biology of type II fatty acid biosynthesis". Annual Review of Biochemistry. 74: 791–831. doi:10.1146/annurev.biochem.74.082803.133524. PMID 15952903.
- ^ Ohlrogge JB, Jaworski JG (June 1997). "Regulation of fatty acid synthesis". Annual Review of Establish Physiology and Plant Molecular Biology. 48: 109–136. doi:x.1146/annurev.arplant.48.1.109. PMID 15012259. S2CID 46348092.
- ^ Grochowski LL, Xu H, White RH (May 2006). "Methanocaldococcus jannaschii uses a modified mevalonate pathway for biosynthesis of isopentenyl diphosphate". Journal of Bacteriology. 188 (ix): 3192–8. doi:10.1128/JB.188.9.3192-3198.2006. PMC1447442. PMID 16621811.
- ^ Lichtenthaler HK (June 1999). "The 1-dideoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants". Annual Review of Institute Physiology and Plant Molecular Biology. 50: 47–65. doi:ten.1146/annurev.arplant.l.one.47. PMID 15012203.
- ^ a b Schroepfer GJ (1981). "Sterol biosynthesis". Annual Review of Biochemistry. 50: 585–621. doi:10.1146/annurev.bi.fifty.070181.003101. PMID 7023367.
- ^ Lees ND, Skaggs B, Kirsch DR, Bard Grand (March 1995). "Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae--a review". Lipids. 30 (3): 221–half-dozen. doi:x.1007/BF02537824. PMID 7791529. S2CID 4019443.
- ^ a b Bhagavan NV (2002). Medical Biochemistry. San Diego: Harcourt/Academic Printing. ISBN978-0-12-095440-7.
- ^ Russo GL (March 2009). "Dietary n-six and n-iii polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention". Biochemical Pharmacology. 77 (6): 937–46. doi:10.1016/j.bcp.2008.x.020. PMID 19022225.
- ^ Riediger ND, Othman RA, Suh K, Moghadasian MH (Apr 2009). "A systemic review of the roles of n-3 fat acids in wellness and disease". Journal of the American Dietetic Association. 109 (iv): 668–79. doi:10.1016/j.jada.2008.12.022. PMID 19328262.
- ^ Galli C, Risé P (2009). "Fish consumption, omega iii fat acids and cardiovascular disease. The science and the clinical trials". Nutrition and Health. twenty (one): 11–xx. doi:ten.1177/026010600902000102. PMID 19326716. S2CID 20742062.
- ^ Micha R, Mozaffarian D (2008). "Trans fatty acids: furnishings on cardiometabolic wellness and implications for policy". Prostaglandins, Leukotrienes, and Essential Fatty Acids. 79 (3–5): 147–52. doi:ten.1016/j.plefa.2008.09.008. PMC2639783. PMID 18996687.
- ^ Dalainas I, Ioannou HP (Apr 2008). "The part of trans fatty acids in atherosclerosis, cardiovascular affliction and infant development". International Angiology. 27 (2): 146–56. PMID 18427401.
- ^ Mozaffarian D, Willett WC (December 2007). "Trans fatty acids and cardiovascular chance: a unique cardiometabolic imprint?". Current Atherosclerosis Reports. 9 (6): 486–93. doi:10.1007/s11883-007-0065-9. PMID 18377789. S2CID 24998042.
- ^ Astrup A, Dyerberg J, Selleck M, Stender S (2008), "Nutrition transition and its relationship to the development of obesity and related chronic diseases", Obes Rev, 9 (S1): 48–52, doi:10.1111/j.1467-789X.2007.00438.x, PMID 18307699, S2CID 34030743
- ^ Astrup A (February 2005). "The role of dietary fat in obesity". Seminars in Vascular Medicine. 5 (i): 40–vii. doi:x.1055/s-2005-871740. PMID 15968579.
- ^ Astrup A (2008). "Dietary direction of obesity". Journal of Parenteral and Enteral Nutrition. 32 (5): 575–vii. doi:10.1177/0148607108321707. PMID 18753397.
- ^ Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. (Feb 2006). "Depression-fat dietary pattern and risk of colorectal cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial". Journal of the American Medical Association. 295 (6): 643–54. doi:10.1001/jama.295.six.643. PMID 16467233.

- ^ Howard BV, Manson JE, Stefanick ML, Beresford SA, Frank G, Jones B, Rodabough RJ, Snetselaar L, Thomson C, Tinker L, Vitolins M, Prentice R (Jan 2006). "Low-fat dietary blueprint and weight change over vii years: the Women'due south Health Initiative Dietary Modification Trial". Periodical of the American Medical Association. 295 (one): 39–49. doi:ten.1001/jama.295.ane.39. PMID 16391215.

- ^ "The Nutrition Source". T. H. Chan School of Public Health. Harvard University.
- ^ "Fats and Cholesterol: Out with the Bad, In with the Proficient — What Should You Eat? – The Diet Source". Harvard Schoolhouse of Public Health.
Bibliography [edit]
- Bhagavan NV (2002). Medical Biochemistry. San Diego: Harcourt/Academic Press. ISBN978-0-12-095440-7.
- Devlin TM (1997). Textbook of Biochemistry: With Clinical Correlations (4th ed.). Chichester: John Wiley & Sons. ISBN978-0-471-17053-ii.
- Stryer L, Berg JM, Tymoczko JL (2007). Biochemistry (6th ed.). San Francisco: W.H. Freeman. ISBN978-0-7167-8724-2.
- van Holde KE, Mathews CK (1996). Biochemistry (2nd ed.). Menlo Park, California: Benjamin/Cummings Pub. Co. ISBN978-0-8053-3931-4.
External links [edit]
| | Look up lipid in Wiktionary, the complimentary dictionary. |
| | Wikimedia Commons has media related to Lipids. |
Introductory
- List of lipid-related spider web sites
- Nature Lipidomics Gateway – Round-upwardly and summaries of recent lipid research
- Lipid Library – General reference on lipid chemistry and biochemistry
- Cyberlipid.org – Resources and history for lipids.
- Molecular Calculator Simulations – Modeling of Lipid Membranes
- Lipids, Membranes and Vesicle Trafficking – The Virtual Library of Biochemistry, Molecular Biology and Prison cell Biology
Nomenclature
- IUPAC classification of lipids
- IUPAC glossary entry for the lipid course of molecules
Databases
- LIPID MAPS – Comprehensive lipid and lipid-associated gene/protein databases.
- LipidBank – Japanese database of lipids and related properties, spectral data and references.
General
- ApolloLipids – Provides dyslipidemia and cardiovascular disease prevention and handling information as well as continuing medical education programs
- National Lipid Association – Professional medical education organization for wellness care professionals who seek to prevent morbidity and mortality stemming from dyslipidemias and other cholesterol-related disorders.
Which Statements About The Makeup Of Biological Membranes Are True?,
Source: https://en.wikipedia.org/wiki/Lipid
Posted by: printzdierack65.blogspot.com


0 Response to "Which Statements About The Makeup Of Biological Membranes Are True?"
Post a Comment